We are studying various interfacial gas (mainly CO2) separation, carbon utilization, and carbon storage research under various temperature and pressure conditions. By leveraging microfluidic techniques, we have attempted to provide cost-effective, fast, and environmentally benign approaches for carbon dissolution (or capture) in aqueous solutions when compared with conventional largescale experiments. We hope that our findings help protect our environment and ecosystems with better strategies. Examples of our research topics include:
1) CO2 diffusivity measurements into water 2) Visualization of three-phase flow (CO2-water-solid precipitation) in porous media 3) CO2 Capture by ethanolamine-nanoparticles 4) CO2 Hydration with polymer-nanoparticles 5) Biofuel – Improved Efficiency for Growth & Separation
1) CO2 Diffusivity Measurements
Measurements of accurate CO2 diffusivity to water are important to predict geological CO2 storage capacity as well as to secure safe/permanent storage strategies into saline formations. Images below are representatives of experimental setups and corresponding results.
aaaaaaaaaaaaaaaaaa <CO2 Diffusivity Measurement Setup>
<CO2 Diffusivity Measurement Setup>
aaaaaaaaaaaaaaaaaa <Data Analysis and Image Processing>
<Data Analysis and Image Processing>
2) Visualization of Three-Phase Flow in Porous Media
<CO2 diffusion to brine>
a
Solid precipitations during CO2 injection in saline aquifers, porous geological formations filled with brine and several minerals, located 1-3 km underground, cause serious problems such as the increase of injection cost, the decrease of overall injectivity, and artificial earthquakes due to high-pressure operations. Proper understanding of CO2 injection processes into porous media will help to resolve these hard questions. The video and images below are examples of experimental results including the chip fabrication procedure.
<CO2 injection to porous media>
3) CO2 Capture by Ethanolamine-Nanoparticles
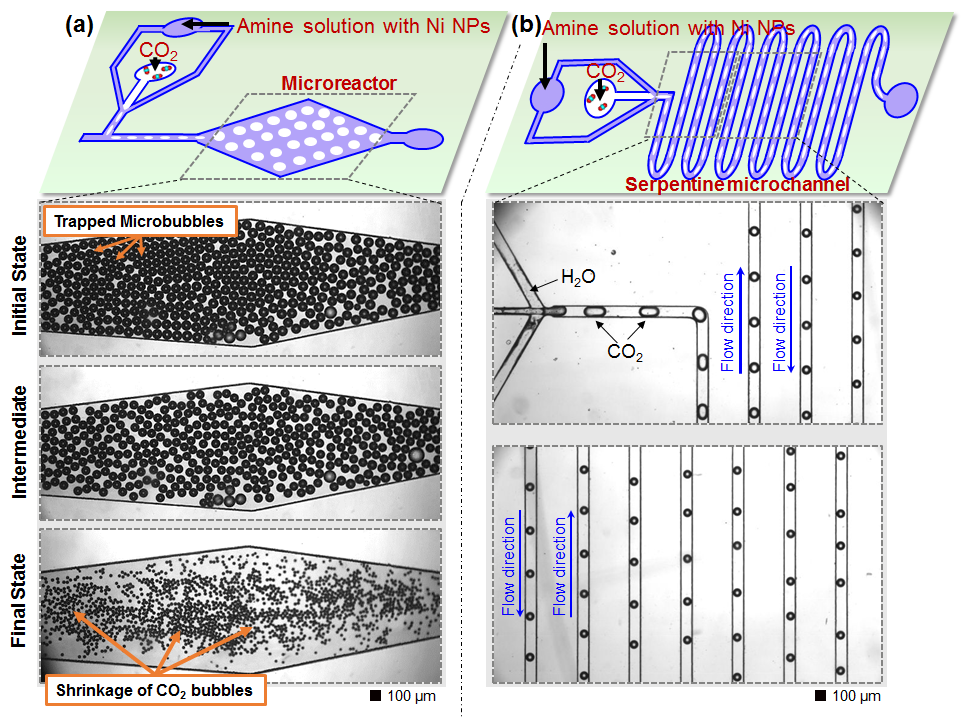
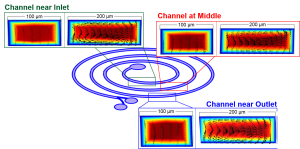
In industrial post-carbon capture processes, monoethanolamine (MEA) has been mainly used as an absorption solvent. However, this approach generates significant amounts of toxic wastewater containing a heavy chemical difficult to treat and also raises concerns about acute corrosion of metal structures in the facility. To reduce the use of MEA in carbon capture, this work evaluates the catalytic performance of nickel nanoparticles (NiNPs) for CO2 capture as a possible additive in an MEA solvent. We test the CO2 absorption rate in MEA catalyzed by NiNPs in both limited and high mixing conditions to model real capturing processes in the packed column of industrial absorption reactors. For this purpose, a microreactor and a long serpentine microchannel are employed. The catalytic absorption performance of NiNPs for CO2 in aqueous MEA is evaluated using CO2 microbubbles by monitoring changes in size upon their time-dependent absorption. We find that the average CO2 absorption rate with NiNPs is accelerated by 34% in the limited mixing condition in the microreactor. This increase is mainly due to NPs’ catalytic CO2 absorption driven by a Brownian motion. On the other hand, in the high mixing condition in the long serpentine microchannel, the catalytic activity of NiNPs improves the average CO2 absorption rate further to 54%. This improvement makes it possible to shorten the timescale for reaching CO2 absorption equilibrium and therefore to reduce the size of the reactors significantly. The test results demonstrate that NiNPs serve as suitable additives in the MEA-based CO2 absorption system.
4) CO2 Hydration with Polymer-Nanoparticles
5) Biofuel – Improved Efficiency for Growth & Separation